Skip to product information


Bioenergy Healthcare Gmbh
Beh Macular Capsules 28 cap
€36,90
Shipping calculated at checkout.
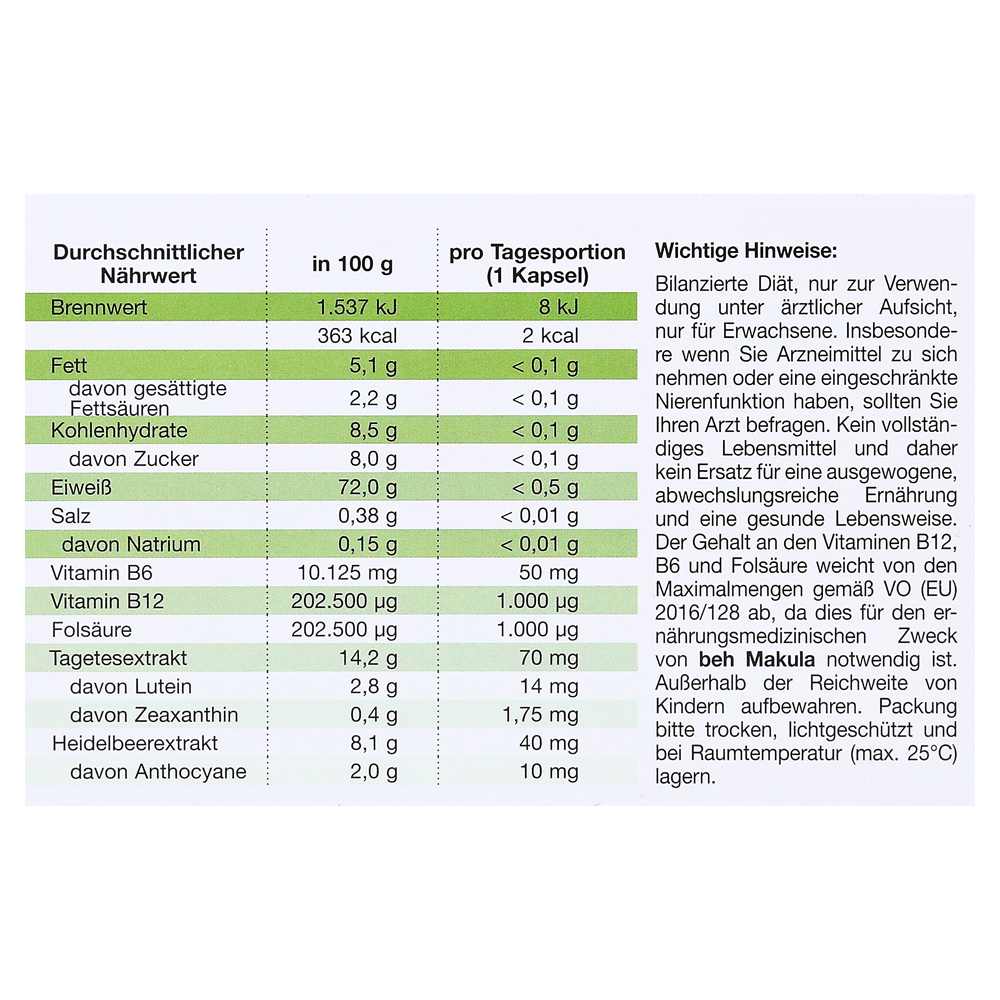
Beh Macular Capsules for the dietary treatment of age-related macular degeneration
beh Makula contains a selection of valuable micronutrients for nutritional purposes and is adapted to the special nutritional requirements of patients with AMD and homocysteine-associated circulatory disorders of the eye.